Pharmaceuticals domain

Social issues and businesses relevant to the Pharmaceuticals domain
Responding to unmet medical needs
Responding to patient-centered healthcare needs
-

Crysvita
We started selling this product in North America and expanded our marketing in other areas, resulting in Crysvita reaching 6,000 patients in 2023. We aim to promote disease awareness activities in line with evidence to further grow its value.
-

Poteligeo
Revenue grew steadily and we expanded markets to countries and regions within EMEA/Asia. Moving forward, we will continue to evolve promotion activities by utilizing evidence to promote the permeation of Poteligeo in existing markets and expanding its targets.
-
KHK4083
(Generic name: rocatinlimab)Currently, eight kinds of phase 3 (testing), ROCKET programs, are underway. Additionally, we are currently performing clinical trials to test the product’s effectiveness against asthma and nodular prurigo in order to expand the product’s target diseases in 2024. We will continue to accelerate its global development.
Business examples in the Pharmaceuticals domain:
Building a Structure and System
That Continually Creates Groundbreaking, New Drugs
-

Director, Senior Managing Executive Officer
Kyowa Kirin Co., Ltd.Takeyoshi Yamashita
Strengthening ability to meet needs by acquiring Orchard Therapeutics
Our business, of which the production and selling of new medicines are the foundation, contributes to society through evolving health care by creating and providing new medicines that surpass existing treatments. However, the difficulty of creating new medicines and R&D expenses continue to grow. Furthermore, the economic pressure felt from skyrocketing medical expenses has become a global issue, and countries are promoting policies and regulations to lower the prices of pharmaceuticals. In recent years, the concept of value-based pricing, in which prices are set equal to the value provided, for pharmaceuticals has been expanding, requiring CSV management that realizes both social and economic value. We have turned our eyes to diseases without treatments and patients who need new medicines and formulated Vision 2030, which focuses on providing life-changing value*1 through pharmaceuticals with real value.
Crysvita and Poteligeo, two of our products, have been accepted by society as pharmaceuticals that provide real life-changing value and have been at the core of the global expansion of our business. We are now promoting the development of a product expected to drive our future growth: rocatinlimab. Through these efforts, we will build a foundation toward achieving Vision 2030 and steadily move forward. Meanwhile, in regard to 2030 and beyond, we must strengthen our ability to meet future needs. Therefore, we have chosen Orchard Therapeutics, a company that fit with our vision and will contribute to our further growth.
There were two reasons for acquiring Orchard Therapeutics. The first reason was to enhance products with life-changing value that can meet unmet medical needs*2 and the development pipeline. The other reason was to secure hematopoietic stem cell gene therapy, a stepping stone to cellular gene therapy that will challenge fundamental treatments, and the relative R&D platform.
Accelerating research on cellular medicine, which has recently entered the spotlight
Orchard Therapeutics is leading the implementation of hematopoietic stem cell gene therapy, and they have already launched Libmeldy in Europe with plans to release it in the United States in 2024. Libmeldy is the first pharmaceutical for metachromatic leukodystrophy, an extremely serious disease, and it provides treatment that can only be achieved with hematopoietic stem cell gene therapy technology. The number of patients who can use this medicine is extremely small, but it is accepted at a high price. Additionally, the clinical development of OTL-203 and OTL-201, two products that also provide life-changing value, is now underway, and the core of the cellular gene therapy business is being established. By adding this business to our global businesses, we can boost our presence as a Global Specialty Pharmaceutical Company that provides life-changing value and take another step toward achieving Vision 2030.
Hematopoietic stem cell gene therapy technology, the specialty of Orchard Therapeutics, isolates the patient’s blood stem cells, the source of various blood cells, manipulates the genes, and then returns them to the patient’s body. The various technologies at each important step are not only excellent, but their strength is that they are ready for practical use. Modern products and developments target congenital diseases caused by a single genetic abnormality, but this platform has development potential, and we believe that combining it with the biotechnology and antibody technology that we have cultivated over the years will widen the scope of its application.
In addition, we can accelerate the research of cellular medicine, which has recently entered the spotlight as a technology that can make possible treatments that are impossible for conventional pharmaceuticals. First, we will aim to realize Vision 2030 by starting to provide unprecedented treatments for patients suffering from serious genetic disorders. Going beyond 2030, we will continue to promote R&D in order to provide solutions to unmet medical needs.
Leveraging innovative modality to develop pharmaceuticals
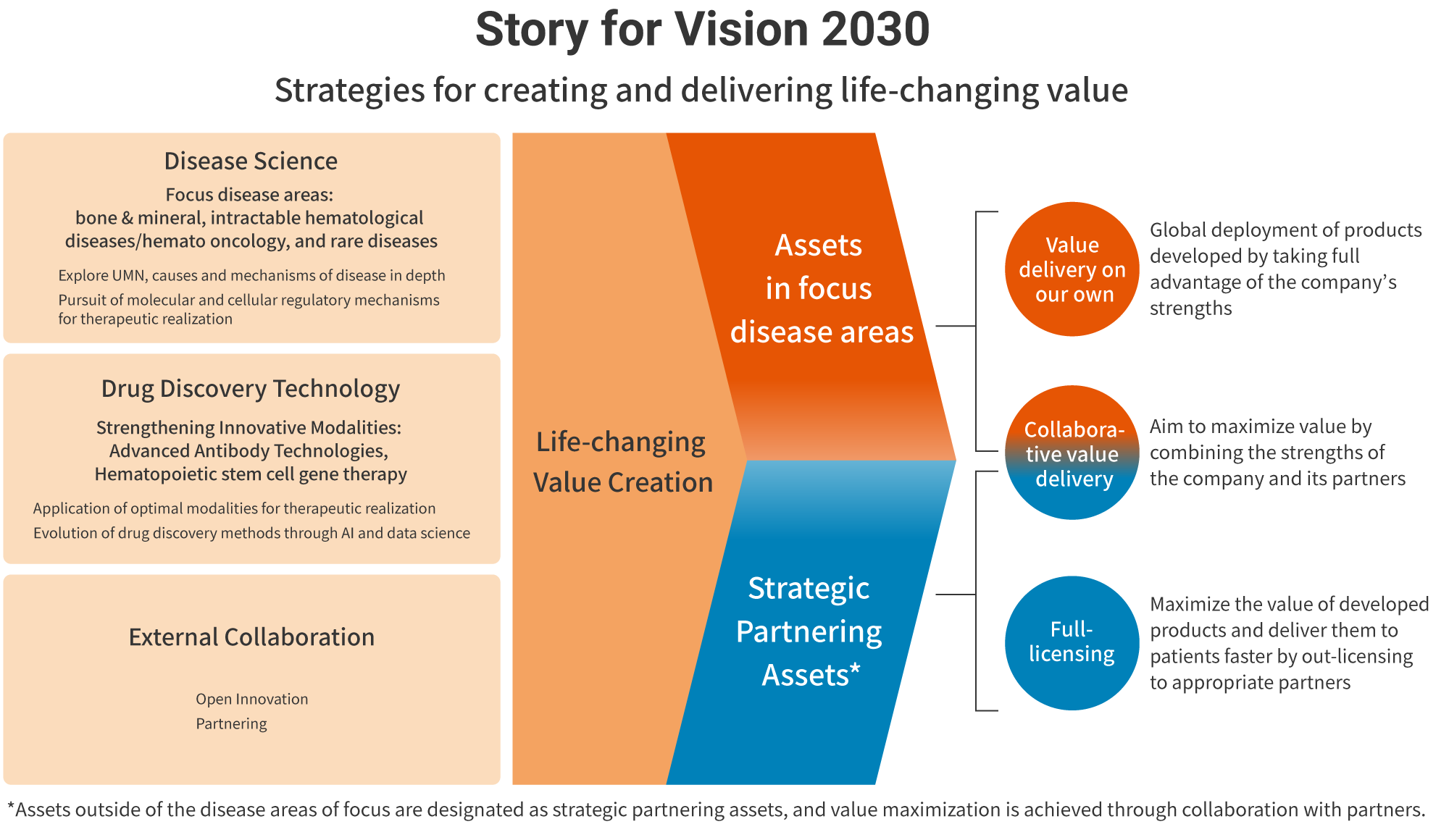
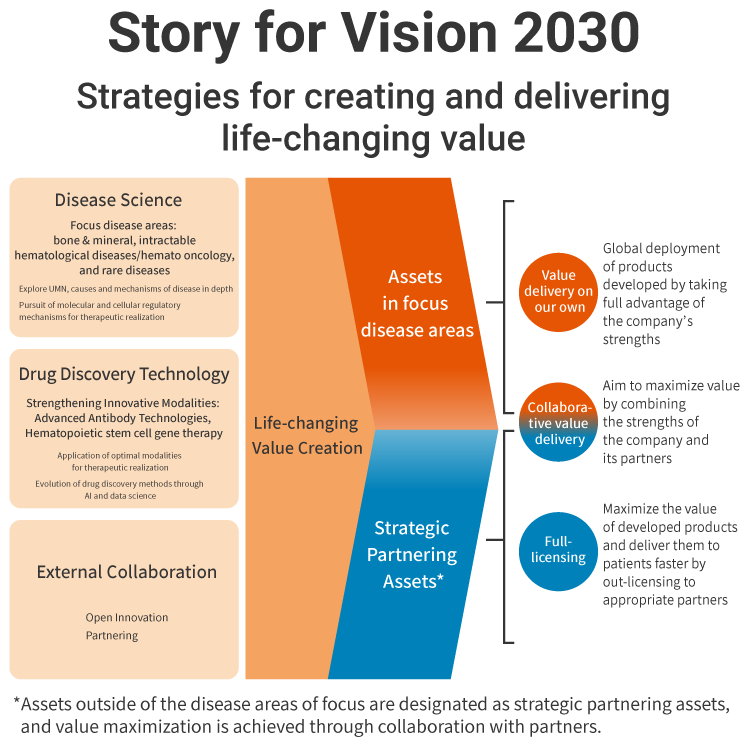
Thanks to the success of Crysvita and Poteligeo over the last three years, we established our global sales structure. We are also steadily promoting the commercialization and maximization of the value of rocatinlimab in collaboration with a major pharmaceutical company and are achieving growth based on our ability to create value. Our image of a Global Specialty Pharmaceutical Company and process of achieving Vision 2030 have become significantly more concrete than when the Medium-Term Business Plan was formulated in 2020, and we revealed it in February 2024 as the Story for Vision 2030. We introduce the contents below.
The creation of life-changing value is the source of our competitive strength, and we are continuously striving to enhance our R&D capabilities for creating solutions to unmet medical needs. As this is a knowledge-intensive industry, we will focus on bones & minerals, intractable hematological diseases/hemato oncology, and rare diseases as strategic domains in which we cultivate our strengths, while aiming to develop pharmaceuticals that realize advanced treatments by utilizing the innovative modalities of advanced antibody and cellular gene technologies.
In focus domains, we will sell our own products globally, but we will leverage strategic partnering in domains that we cannot cover on our own in order to provide value to more patients.
We are committed to getting closer with patients and to realizing a business that sustainably creates and provides life-changing value in line with Story for Vision 2030.
- The value that is produced by identifying unmet medical needs of sick people, imagining new medicines and services to resolve those issues, and then providing them so that patients can feel a dramatic improvement in their lives and smile.
- Medical needs that are unresolved